Create articles from any YouTube video or use our API to get YouTube transcriptions
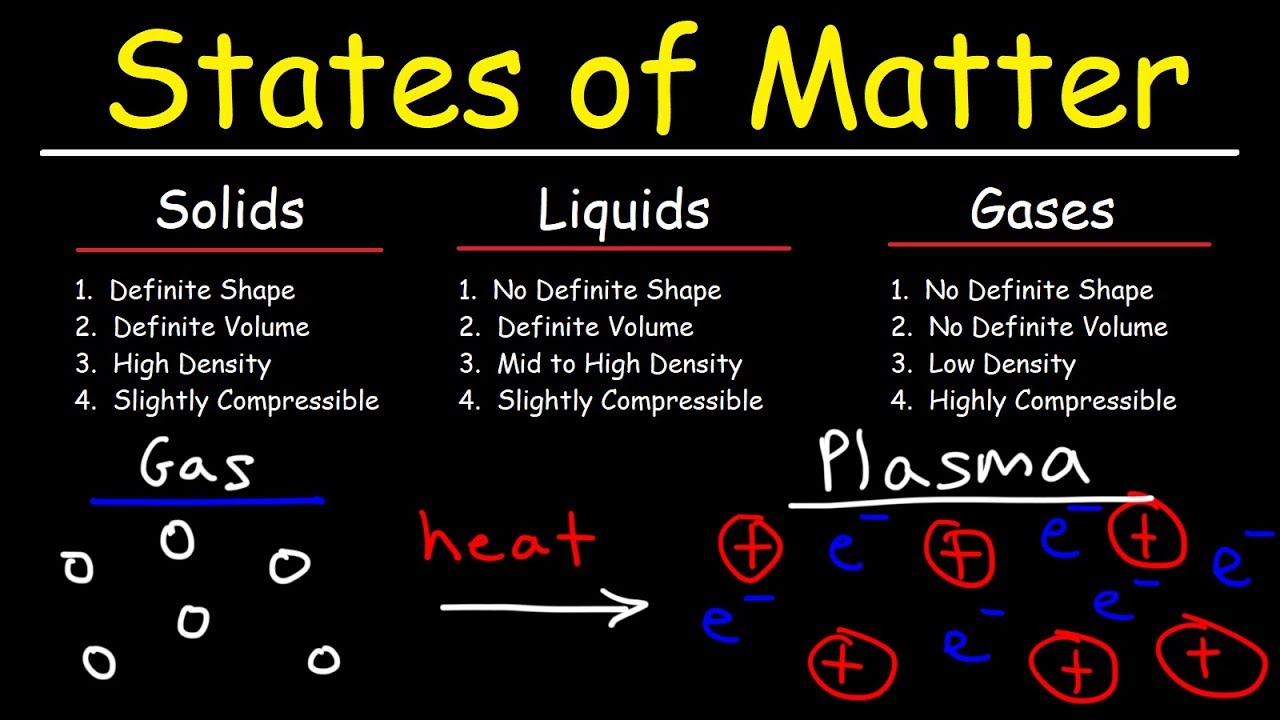
Start for freeMatter's ability to change its state—from solid to liquid to gas—is a phenomenon that has intrigued scientists and laypeople alike. This transformation, known as a phase change, is not just a macroscopic event but involves intricate changes at the atomic and molecular levels. In this exploration, we'll delve into what happens inside matter during a phase change and how the particles of matter behave as they transition from one state to another.
The Transformation from Solid to Liquid
Imagine a beaker filled with ice, with a thermometer attached, being heated. As heat is applied, the ice begins to melt. This process is not just about the ice turning into water; it's about understanding what happens on a microscopic scale.
Particle Movement and Kinetic Energy
Initially, the ice particles are closely packed and vibrate slightly in their positions. Upon heating, their kinetic energy increases, causing them to vibrate more vigorously. This increase in kinetic energy eventually leads to the particles breaking the forces of attraction binding them, allowing them to move more freely. This marks the transition from solid (ice) to liquid (water).
Melting Point and Temperature Stability
An interesting observation during the melting process is that the temperature remains constant at 0 degrees Celsius. This temperature, known as the melting point, is crucial because it signifies the minimum temperature at which a solid begins to turn into a liquid. For ice, the melting point is at 0 degrees Celsius, but other substances have different melting points, indicating the strength of the intermolecular forces within them. Even as heat continues to be applied, the temperature does not rise until all the ice has melted. The energy supplied goes into breaking the intermolecular forces rather than increasing the temperature.
The Transition from Liquid to Gas
Upon further heating, the liquid water's temperature rises until it reaches the boiling point, at which water begins to evaporate into water vapor. Boiling is a process that occurs throughout the liquid, indicating a change from liquid to gas.
Boiling Point and Kinetic Energy Increase
The boiling point, similar to the melting point, is the minimum temperature at which a liquid changes into a vapor. For water, this is 100 degrees Celsius. As heat is supplied, the particles in the liquid gain more kinetic energy, breaking the forces of attraction between them and transitioning into a gas state.
The Role of Pressure in Phase Changes
Temperature is not the only factor that affects the state of matter; pressure plays a significant role as well. By manipulating pressure, we can cause gases to liquefy or even solidify. An example of this is solid carbon dioxide (dry ice), which under high pressure solidifies and, when exposed to atmospheric pressure, sublimates directly into gas. Decreasing the volume of a container with gas by pushing down a piston, for instance, increases pressure and can lead to the liquefaction of the gas, provided the temperature is kept low.
Increasing pressure brings particles closer together, enhancing the intermolecular forces and facilitating the transition from gas to liquid. This principle is not only fascinating from a scientific standpoint but also has practical applications in various industries, including the manufacture of liquid fuels and the storage of gases.
In conclusion, the transformation of matter from one state to another is a complex process influenced by temperature and pressure. It reflects the dynamic nature of particle interactions and the balance between kinetic energy and intermolecular forces. Understanding these changes provides insight into the fundamental principles of physics and chemistry that govern the natural world.
Explore more about the wonders of phase changes and the science behind them in this detailed video: Understanding Phase Changes in Matter.