Create articles from any YouTube video or use our API to get YouTube transcriptions
Start for freeIn the realm of physics, understanding the states of matter is fundamental to grasping how the world around us behaves. Matter, essentially anything that has mass and occupies space, is composed of minuscule particles called atoms. These atoms come together to form the states of matter we interact with daily: solids, liquids, and gases, with plasma as an additional, less commonly encountered state. This article delves into these states, their characteristics, and the transitions between them, offering insights into the fascinating world of matter.
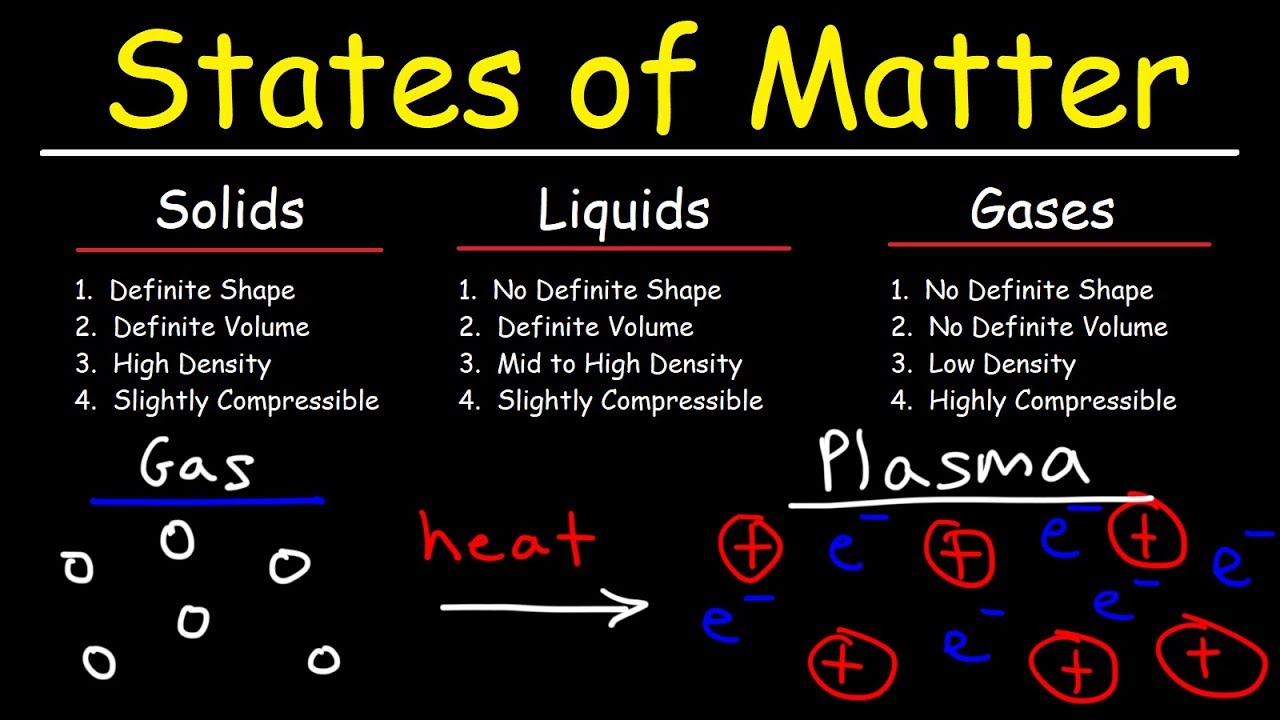
Solids, Liquids, and Gases: The Basics
Solids
A solid is characterized by its definite shape and volume. Think of a rock; its shape and volume remain constant regardless of where it is placed. This rigidity is due to the closely packed atoms within solids, which do not have the ability to flow.
Liquids
Unlike solids, liquids have a definite volume but no definite shape. They take the shape of their container, flowing to fit its form. This property is because the atoms in liquids are less tightly packed than in solids, allowing them to move around more freely. Liquids can vary in viscosity, with some flowing easily like water, while others, like honey, move more slowly.
Gases
Gases have neither a definite shape nor a definite volume, filling any container they are placed in. They can flow through spaces, like liquids, but are much less dense. This low density also makes gases highly compressible, a feature not shared with solids or liquids due to their closely packed atoms.
Understanding Density
Density, defined as mass divided by volume, varies across the states of matter. Solids generally have the highest density, followed by liquids, and gases have the lowest. For example, the density of air at 20 degrees Celsius is about 1.2 kilograms per cubic meter, significantly lower than that of liquid water at 1000 kilograms per cubic meter. An interesting exception to the rule that solids are denser than liquids is ice, which is less dense than liquid water.
Phase Changes: The Transitions
Matter can transition between states through the process of phase changes, which occur when heat is added or removed. Adding heat to a solid, for instance, can melt it into a liquid, and further heating can turn the liquid into a gas. Conversely, cooling a gas can condense it into a liquid, and further cooling can freeze the liquid into a solid. These transformations illustrate the dynamic nature of matter and its responsiveness to temperature changes.
Key Phase Change Terms
- Melting: Solid to liquid
- Vaporization: Liquid to gas
- Sublimation: Solid directly to gas (e.g., dry ice)
- Freezing: Liquid to solid
- Condensation: Gas to liquid
- Deposition: Gas directly to solid
The Fourth State: Plasma
Plasma, an ionized gas, is a state of matter where the gas is heated to such a degree that some electrons are stripped away from their atoms, creating a mixture of ions and free electrons. This state conducts electricity and is what makes neon signs glow and the sun shine. Plasma's unique properties distinguish it from the other states of matter, showcasing the diversity and complexity of the material world.
From the solidity of rocks to the fluidity of water and the expansiveness of air, the states of matter shape our perceptions and interactions with the environment. By exploring the principles of density, phase changes, and the existence of plasma, we gain a deeper appreciation for the material complexity of the universe. Whether in the classroom or the laboratory, the study of matter and its transformations remains a fundamental aspect of understanding the physical world.
For more educational content on the states of matter and other fascinating topics, don't forget to subscribe to our channel. Watch the full video here.


